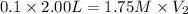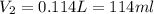Chemistry, 03.10.2019 11:30 JvGaming2001
Astudent requires 2.00 l of 0.100 m nh4no3 from a 1.75 m nh4no3 stock solution. what is the correct way to get the solution?
use mc021-1.jpg.
measure 114 ml of the 1.75 m solution, and dilute it to 1.00 l.
measure 114 ml of the 1.75 m solution, and dilute it to 2.00 l.
measure 8.75 ml of the 1.75 m solution, and dilute it to 2.00 l.
measure 8.75 ml of the 0.100 m solution, and dilute it to 2.00 l.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1
You know the right answer?
Astudent requires 2.00 l of 0.100 m nh4no3 from a 1.75 m nh4no3 stock solution. what is the correct...
Questions




English, 01.09.2020 19:01

History, 01.09.2020 19:01


Mathematics, 01.09.2020 19:01


History, 01.09.2020 19:01






Social Studies, 01.09.2020 19:01




Biology, 01.09.2020 19:01

Mathematics, 01.09.2020 19:01

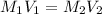
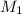 = molarity of first solution
= molarity of first solution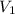 = Volume of first solution
= Volume of first solution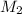 = molarity of second solution
= molarity of second solution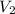 = Volume of second solution
= Volume of second solution