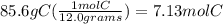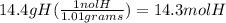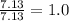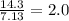Chemistry, 30.10.2019 16:31 Queenhagar
What is the empirical formula of a compound that is 85.6% carbon and 14.4% hydrogen by weight?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
What is the empirical formula of a compound that is 85.6% carbon and 14.4% hydrogen by weight?...
Questions

Mathematics, 09.02.2021 02:10

Mathematics, 09.02.2021 02:10



English, 09.02.2021 02:10


History, 09.02.2021 02:10

Engineering, 09.02.2021 02:10










Mathematics, 09.02.2021 02:10