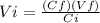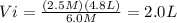Chemistry, 04.01.2020 15:31 llamasking
Alaboratory experiment requires 4.8 l of a 2.5 m solution of sulfuric acid (h2so4), but the only available h2so4 is a 6.0 m stock solution. how could you prepare the solution needed for the lab experiment? show all the work used to find your answer.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1


Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Alaboratory experiment requires 4.8 l of a 2.5 m solution of sulfuric acid (h2so4), but the only ava...
Questions

Mathematics, 05.12.2019 16:31

English, 05.12.2019 16:31


History, 05.12.2019 16:31




Mathematics, 05.12.2019 16:31

English, 05.12.2019 16:31


Mathematics, 05.12.2019 16:31

Computers and Technology, 05.12.2019 16:31

Mathematics, 05.12.2019 16:31


English, 05.12.2019 16:31

Computers and Technology, 05.12.2019 16:31


Computers and Technology, 05.12.2019 16:31


Mathematics, 05.12.2019 16:31