
Chemistry, 03.10.2019 08:00 student679
C-12 and c-13 are naturally occurring isotopes of the element carbon. c-12 occurs 98.89% of the time and c-13 occurs 1.108% of the time. what calculation should be used to determine the atomic mass of this element?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1


Chemistry, 22.06.2019 09:00
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1
You know the right answer?
C-12 and c-13 are naturally occurring isotopes of the element carbon. c-12 occurs 98.89% of the time...
Questions

Mathematics, 07.10.2019 10:30

History, 07.10.2019 10:30




Mathematics, 07.10.2019 10:30

History, 07.10.2019 10:30



Chemistry, 07.10.2019 10:30


Social Studies, 07.10.2019 10:30






Mathematics, 07.10.2019 10:30

Mathematics, 07.10.2019 10:30

Computers and Technology, 07.10.2019 10:30

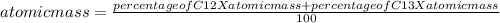
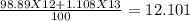 g/mol
g/mol

