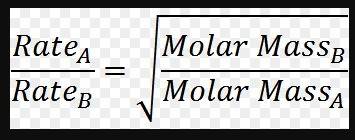In a given sample, hydrogen gas effuses four times faster than the other gas present in the mixture. what is the molar mass and identity of the unknown gas if the molar mass of hydrogen is 2 grams? 16 grams and oxygen 32 grams and oxygen 8 grams and nitrogen 4 grams and helium 64 grams and ozone

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
In a given sample, hydrogen gas effuses four times faster than the other gas present in the mixture....
Questions

English, 17.03.2020 17:57





Biology, 17.03.2020 17:57

Mathematics, 17.03.2020 17:57

Geography, 17.03.2020 17:57


Mathematics, 17.03.2020 17:58




Chemistry, 17.03.2020 17:58