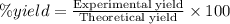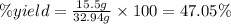One method to produce nitrogen in the lab is to react ammonia with copper (ii) oxide: nh3(
g....

Chemistry, 30.09.2019 12:00 Juliette9525
One method to produce nitrogen in the lab is to react ammonia with copper (ii) oxide: nh3(
g. + cuo(s) cu(s) + h2o(l) + n2(
g. after using 40.0 grams of nh3, 15.5 grams of n2 are produced. what is the percent yield of nitrogen in the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
One significant difference between an ionic bond, where electrons are taken from one atom and added to another atom, and a covalent or metallic bond, where electrons are shared is
Answers: 2

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2
You know the right answer?
Questions





Mathematics, 01.06.2021 20:30


Mathematics, 01.06.2021 20:30


Mathematics, 01.06.2021 20:30

Spanish, 01.06.2021 20:30

Social Studies, 01.06.2021 20:30

Mathematics, 01.06.2021 20:30

History, 01.06.2021 20:30


Mathematics, 01.06.2021 20:30

Mathematics, 01.06.2021 20:30

English, 01.06.2021 20:30



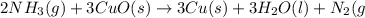
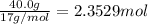
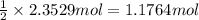 of nitrogen gas
of nitrogen gas