
Chemistry, 24.11.2019 22:31 fortwill05
Rust results from iron’s reaction to oxygen. an iron nail gains mass when it rusts. how does this reaction support the law of conservation of mass?
a. the mass of the rusted nail equals the mass of iron and the oxygen from the air it reacted with to form the rust.
b. the mass of the rusted nail increases because iron attracts more protons from the air.
c. the increased mass of the rusted nail is an exception to the law of conservation of mass since the rusted nail’s mass increases.
d. the increased mass of the rusted nail results from the rearrangement of protons and neutrons within oxygen, according to the law of conservation of mass.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1
You know the right answer?
Rust results from iron’s reaction to oxygen. an iron nail gains mass when it rusts. how does this re...
Questions

Mathematics, 26.08.2019 11:30

Mathematics, 26.08.2019 11:30

History, 26.08.2019 11:30


Spanish, 26.08.2019 11:30

Mathematics, 26.08.2019 11:30

English, 26.08.2019 11:30


Social Studies, 26.08.2019 11:30


Physics, 26.08.2019 11:30





Mathematics, 26.08.2019 11:30


Mathematics, 26.08.2019 11:30


Social Studies, 26.08.2019 11:30

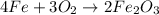
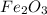 .
.

