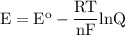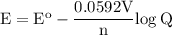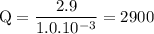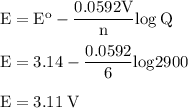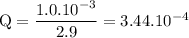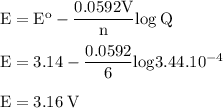Chemistry, 28.09.2019 01:40 hdwoody2002
Answer this question asap. this is on a homework assignment that is due you in advance! a voltaic cell employs the following redox reaction: 2fe3+(aq)+3mg(s)→2fe(s)+3mg2+(aq) calculate the cell potential at 25 ∘c under each of the following conditions.
a. find ecell at standard conditions
b. when [fe3+]= 1.0×10−3 m ; [mg2+]= 2.90 m find ecell
c. when [fe3+]=2.90m; [mg2+]=1.0*10^-3 m find e cell

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2
You know the right answer?
Answer this question asap. this is on a homework assignment that is due you in advance! a voltaic...
Questions

History, 14.07.2019 18:30




Arts, 14.07.2019 18:30



Physics, 14.07.2019 18:30

Geography, 14.07.2019 18:30


History, 14.07.2019 18:30



World Languages, 14.07.2019 18:30


History, 14.07.2019 18:30