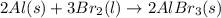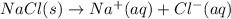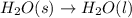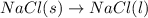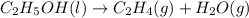Chemistry, 29.09.2019 05:30 rissacoob7862
Which of the following processes would you expect to have a negative value for entropy? nacl(s) na+(aq) + cl- (aq) h2o(s) h2o(l) nacl(s) nacl(l) 2 al(s) + 3br2(l) 2albr3(s) c2h5oh(l) c2h4(g) + h2o(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2
You know the right answer?
Which of the following processes would you expect to have a negative value for entropy? nacl(s) na+...
Questions



Chemistry, 03.03.2021 03:20

Mathematics, 03.03.2021 03:20


Mathematics, 03.03.2021 03:20


Arts, 03.03.2021 03:20

Biology, 03.03.2021 03:20

Physics, 03.03.2021 03:20


Mathematics, 03.03.2021 03:20



Mathematics, 03.03.2021 03:20

Mathematics, 03.03.2021 03:20

Social Studies, 03.03.2021 03:20



Social Studies, 03.03.2021 03:20