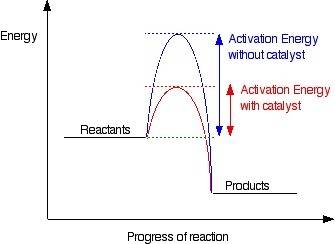
How do catalysts increase the rate of a chemical reaction?
they lower the activation energy.<...

Chemistry, 23.01.2020 10:31 christianmason9423
How do catalysts increase the rate of a chemical reaction?
they lower the activation energy.
they lower the collision energy.
they increase the collision energy.
they increase the temperature.
they increase the concentration of reactants.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

You know the right answer?
Questions

Mathematics, 02.12.2020 22:40

English, 02.12.2020 22:40



Mathematics, 02.12.2020 22:40


Mathematics, 02.12.2020 22:40

Chemistry, 02.12.2020 22:40

Mathematics, 02.12.2020 22:40

History, 02.12.2020 22:40

Mathematics, 02.12.2020 22:40

Mathematics, 02.12.2020 22:40


Physics, 02.12.2020 22:40

Mathematics, 02.12.2020 22:40


Social Studies, 02.12.2020 22:40



Mathematics, 02.12.2020 22:40