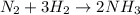Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1

Chemistry, 23.06.2019 10:10
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3

Chemistry, 23.06.2019 13:30
How many more valence electrons does sodium need to have a full outer valence shell
Answers: 1
You know the right answer?
An industrial process involves reacting nitrogen gas (n2 and hydrogen gas (h2 to produce ammonia (nh...
Questions

Mathematics, 23.02.2021 05:40


Mathematics, 23.02.2021 05:40




History, 23.02.2021 05:40


Mathematics, 23.02.2021 05:40



Social Studies, 23.02.2021 05:40





Computers and Technology, 23.02.2021 05:40

Mathematics, 23.02.2021 05:40


Mathematics, 23.02.2021 05:40