Chemistry, 09.10.2019 19:30 skylar1315
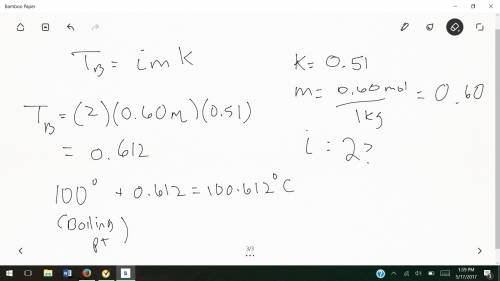
Determine the boiling point of a solution made by dissolving 0.60 mol k2so4 in 1.0 kg water. water has a boiling point elevation constant of 0.51°c•kg/mol. what is the boiling point of this solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1
You know the right answer?
Determine the boiling point of a solution made by dissolving 0.60 mol k2so4 in 1.0 kg water. water h...
Questions



Mathematics, 01.07.2019 18:00


History, 01.07.2019 18:00

Mathematics, 01.07.2019 18:00



Spanish, 01.07.2019 18:00



English, 01.07.2019 18:00



Business, 01.07.2019 18:00


Biology, 01.07.2019 18:00


Chemistry, 01.07.2019 18:00

Spanish, 01.07.2019 18:00