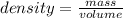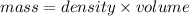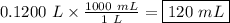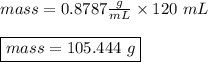Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
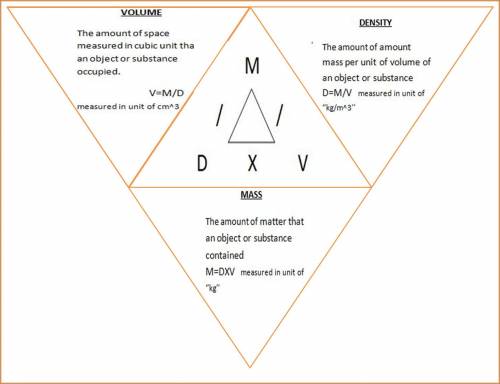
The density of benzene at 15 ∘c is 0.8787 g/ml. calculate the mass of 0.1200 l of benzene at this te...
Questions

Mathematics, 09.09.2020 17:01

Social Studies, 09.09.2020 17:01

Mathematics, 09.09.2020 17:01

Mathematics, 09.09.2020 17:01

Mathematics, 09.09.2020 17:01

English, 09.09.2020 17:01

Chemistry, 09.09.2020 17:01

Mathematics, 09.09.2020 17:01

Mathematics, 09.09.2020 17:01

Mathematics, 09.09.2020 17:01

Mathematics, 09.09.2020 17:01

Mathematics, 09.09.2020 17:01

Social Studies, 09.09.2020 17:01

Social Studies, 09.09.2020 17:01

Mathematics, 09.09.2020 17:01

Mathematics, 09.09.2020 17:01


Mathematics, 09.09.2020 18:01

Computers and Technology, 09.09.2020 18:01