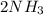Chemistry, 30.09.2019 00:30 markaylarowland8
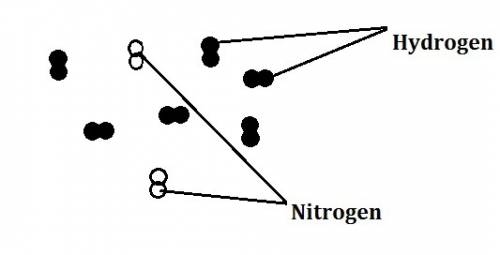
In the haber process, nitrogen (n2) and hydrogen (h2) are directly combined to form ammonia (nh3). which illustration contains the stoichiometric quantities of the reactants for this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1


Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
In the haber process, nitrogen (n2) and hydrogen (h2) are directly combined to form ammonia (nh3). w...
Questions



Mathematics, 08.07.2021 02:10



Mathematics, 08.07.2021 02:10

Mathematics, 08.07.2021 02:10




Mathematics, 08.07.2021 02:10





Biology, 08.07.2021 02:10



Mathematics, 08.07.2021 02:10

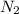 +
+ 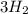 =
=