Chemistry, 05.10.2019 05:20 tanichka2314
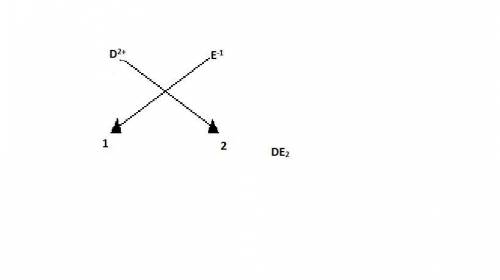
If d+2 would react with e-1, what do you predict the formula to be. a) de b)d2e c)de2 d) d2e2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3


Chemistry, 23.06.2019 08:50
Reacting masses1 calcium carbonate breaks down on heating to produce calcium oxide and carbondioxide gas.caco3 + cao + co2a student heats 15 g of calcium carbonate strongly in a crucible.relative atomic masses (a): ca = 40, c = 12, o = 16.calculate the mass of calcium oxide produced by this reaction.(5 marks)
Answers: 3

Chemistry, 23.06.2019 13:30
How many more valence electrons does sodium need to have a full outer valence shell
Answers: 1
You know the right answer?
If d+2 would react with e-1, what do you predict the formula to be. a) de b)d2e c)de2 d) d2e2...
Questions



Spanish, 18.01.2021 07:40

English, 18.01.2021 07:40



History, 18.01.2021 07:40


Mathematics, 18.01.2021 07:40


Mathematics, 18.01.2021 07:40


Mathematics, 18.01.2021 07:40




Mathematics, 18.01.2021 07:40


Mathematics, 18.01.2021 07:40


 cation and E
cation and E  is an anion with oxidation state of -1. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral
is an anion with oxidation state of -1. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral