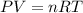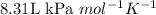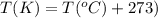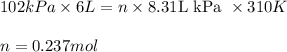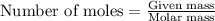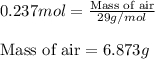Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1
You know the right answer?
An average adult has a total lung capacity of 6.0 l. how many total grams of air could be held in th...
Questions

Mathematics, 05.05.2021 09:20

Health, 05.05.2021 09:20


English, 05.05.2021 09:20



Biology, 05.05.2021 09:20


Mathematics, 05.05.2021 09:20




Physics, 05.05.2021 09:20



Mathematics, 05.05.2021 09:20

Mathematics, 05.05.2021 09:20



Mathematics, 05.05.2021 09:20