
Chemistry, 29.01.2020 08:00 kellynadine02
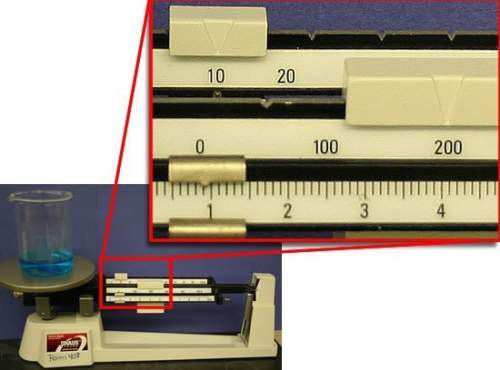
what is the mass of water in the beaker? assume that the beaker has a mass of exactly 100.00 g.
a) 100.90 g
b) 109.00 g
c) 110.90 g
d) 210.90 g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
What is the molecular formula of a hydrocarbon with m+ = 166? (write the formula with no subscripts, e.g. c4h10.) what is the sum of rings and double bonds in this compound?
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
what is the mass of water in the beaker? assume that the beaker has a mass of exactly 100.00 g.
Questions



Social Studies, 21.09.2019 21:00

Mathematics, 21.09.2019 21:00


History, 21.09.2019 21:00

Mathematics, 21.09.2019 21:00

Mathematics, 21.09.2019 21:00






Mathematics, 21.09.2019 21:10

Computers and Technology, 21.09.2019 21:10

History, 21.09.2019 21:10

Mathematics, 21.09.2019 21:10

Mathematics, 21.09.2019 21:10

Physics, 21.09.2019 21:10



