
Chemistry, 01.10.2019 05:00 detrickboucicaut
Acertain liquid has a vapor pressure of 92.0 torr at 23.0 °c and 351.0 torr at 45.0 °c. calculate the value of δh°vap for this liquid.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3
You know the right answer?
Acertain liquid has a vapor pressure of 92.0 torr at 23.0 °c and 351.0 torr at 45.0 °c. calculate th...
Questions


Biology, 14.04.2021 19:40


English, 14.04.2021 19:40

Mathematics, 14.04.2021 19:40

Mathematics, 14.04.2021 19:40


Mathematics, 14.04.2021 19:40



Mathematics, 14.04.2021 19:40


History, 14.04.2021 19:40

English, 14.04.2021 19:40


Mathematics, 14.04.2021 19:40



Mathematics, 14.04.2021 19:40

Chemistry, 14.04.2021 19:40

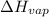 is 47.627 kJ/mol
is 47.627 kJ/mol![\ln(\frac{P_2}{P_1})=\frac{\Delta H_{vap}}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0278/8132/b9a49.png)
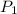 = vapor pressure at temperature
= vapor pressure at temperature 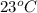 = 92 torr
= 92 torr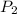 = vapor pressure at temperature
= vapor pressure at temperature 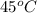 = 351 torr
= 351 torr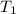 = initial temperature =
= initial temperature = ![23^oC=[23+2730]K=296K](/tpl/images/0278/8132/46201.png)
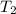 = final temperature =
= final temperature = ![45^oC=[45+2730]K=318K](/tpl/images/0278/8132/1a9ea.png)
![\ln(\frac{351torr}{92torr})=\frac{\Delta H_{vap}}{8.314J/mol.K}[\frac{1}{296}-\frac{1}{318}]\\\\\Delta H_{vap}=47627.347J/mol=47.627kJ/mol](/tpl/images/0278/8132/e398e.png)


