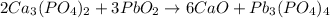Chemistry, 21.11.2019 19:31 joannamarquez0701
When the following reaction is balanced with the lowest possible whole number coefficients, what is the coefficient in front of the calcium oxide (cao)? ca3(po4)2 + pbo2 yields cao + pb3 (po4)4 (2 points) 2 3 6 8

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
When the following reaction is balanced with the lowest possible whole number coefficients, what is...
Questions


Geography, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01


Mathematics, 20.09.2020 01:01


Mathematics, 20.09.2020 01:01




Social Studies, 20.09.2020 01:01

History, 20.09.2020 01:01


Medicine, 20.09.2020 01:01


Social Studies, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01


Biology, 20.09.2020 01:01