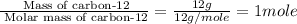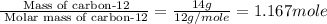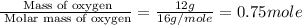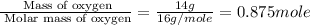Chemistry, 22.09.2019 06:30 andreamarie2004amg
Avogadro's number was calculated by determining the number of atoms in
a. 12.00 g of carbon-12
b. 14.00 g of carbon-12
c. 12.00 g of oxygen
d. 14.00 g of oxygen

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
Avogadro's number was calculated by determining the number of atoms in
a. 12.00 g of carbon-1...
a. 12.00 g of carbon-1...
Questions



Mathematics, 04.03.2021 18:30


Mathematics, 04.03.2021 18:30

History, 04.03.2021 18:30

English, 04.03.2021 18:30



Biology, 04.03.2021 18:30

English, 04.03.2021 18:30







Spanish, 04.03.2021 18:30


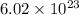 of particles. And this is also known as Avogadro's number.
of particles. And this is also known as Avogadro's number.