
The double-replacement reaction of sulfuric acid with aluminum hydroxide produces aluminum sulfate salt and water. balance this skeleton equation, then determine the mole ratio of h2so4: h2o. h2so4 + al(oh)3 al2(so4)3 + h2o? (how do i determine the mole ration? )
a) 1: 1
b) 2: 3
c) 3: 1
d) 3: 6
e) 6: 3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 23.06.2019 06:40
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1
You know the right answer?
The double-replacement reaction of sulfuric acid with aluminum hydroxide produces aluminum sulfate s...
Questions





Spanish, 30.12.2020 06:20





Mathematics, 30.12.2020 06:20

History, 30.12.2020 06:20




English, 30.12.2020 06:30



Mathematics, 30.12.2020 06:30

English, 30.12.2020 06:30

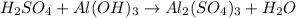
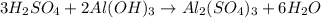
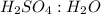 is 3:6.
is 3:6.

