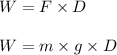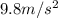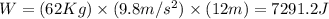Chemistry, 08.10.2019 12:30 peytondavis2424
62 kg box is lifted 12 meters off the ground. how much work was done? a) 5.17 j b) 72.91 j c) 744.0 j d) 7291.2 j

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
You know the right answer?
62 kg box is lifted 12 meters off the ground. how much work was done? a) 5.17 j b) 72.91 j c) 744.0...
Questions

Mathematics, 14.09.2020 17:01

Mathematics, 14.09.2020 17:01

Chemistry, 14.09.2020 17:01

Social Studies, 14.09.2020 17:01

Biology, 14.09.2020 17:01

Mathematics, 14.09.2020 17:01

English, 14.09.2020 17:01

Mathematics, 14.09.2020 17:01

Chemistry, 14.09.2020 17:01

Mathematics, 14.09.2020 17:01

Physics, 14.09.2020 17:01

Mathematics, 14.09.2020 17:01

Mathematics, 14.09.2020 17:01

Mathematics, 14.09.2020 17:01

Mathematics, 14.09.2020 17:01

Mathematics, 14.09.2020 17:01

Mathematics, 14.09.2020 17:01

English, 14.09.2020 17:01

Biology, 14.09.2020 17:01

English, 14.09.2020 17:01