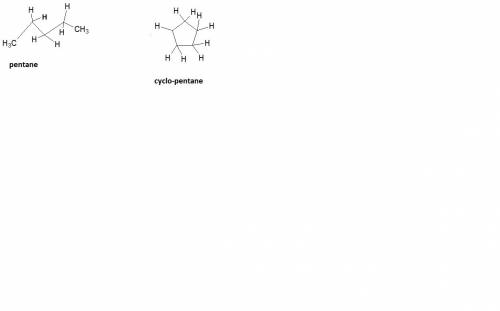
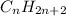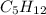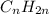How does a straight-chain alkane that has five carbon atoms differ from a cycloalkane that has five carbon atoms?
the straight-chain alkane has at least one double or triple bond, but the cycloalkane has only single bonds.
the straight-chain alkane has only single bonds, but the cycloalkane has at least one double or triple bond.
the straight-chain alkane has two more hydrogen atoms than the cycloalkane.
the straight-chain alkane has two fewer hydrogen atoms than the cycloalkane.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
How does a straight-chain alkane that has five carbon atoms differ from a cycloalkane that has five...
Questions




History, 07.03.2021 04:00

Mathematics, 07.03.2021 04:00

English, 07.03.2021 04:00

History, 07.03.2021 04:00

English, 07.03.2021 04:00


Chemistry, 07.03.2021 04:00




Biology, 07.03.2021 04:00

English, 07.03.2021 04:00

English, 07.03.2021 04:00


Chemistry, 07.03.2021 04:00