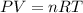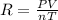Chemistry, 01.09.2019 20:50 tugordochulo1
If the pressure, volume, and the number of moles of a gas are known, which is needed to calculate the universal gas constant from the ideal gas law?
the temperature of the gas
the molar volume of the gas
the molar mass of the gas
the partial pressure of the gas

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

You know the right answer?
If the pressure, volume, and the number of moles of a gas are known, which is needed to calculate th...
Questions



Mathematics, 13.11.2020 19:50


Computers and Technology, 13.11.2020 19:50


History, 13.11.2020 19:50





Mathematics, 13.11.2020 19:50



Mathematics, 13.11.2020 19:50

History, 13.11.2020 19:50

Mathematics, 13.11.2020 19:50


Mathematics, 13.11.2020 19:50