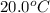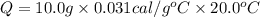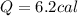Chemistry, 27.01.2020 02:31 chickennuggets0621
The specific heat of gold is 0.031 calories/gram°c. if 10.0 grams of gold were heated and the temperature of the sample changed by 20.0°c, how many calories of heat energy were absorbed by the sample?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Figure 10-1 study figure 10-1. the strong nuclear force felt by a single proton in a large nucleus
Answers: 3

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3
You know the right answer?
The specific heat of gold is 0.031 calories/gram°c. if 10.0 grams of gold were heated and the temper...
Questions

Mathematics, 01.12.2021 16:50


History, 01.12.2021 16:50




Mathematics, 01.12.2021 16:50






Mathematics, 01.12.2021 16:50

Mathematics, 01.12.2021 16:50

Mathematics, 01.12.2021 16:50

SAT, 01.12.2021 16:50


Mathematics, 01.12.2021 16:50

Mathematics, 01.12.2021 16:50

Chemistry, 01.12.2021 16:50

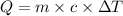
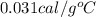
 = change in temperature =
= change in temperature =