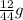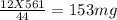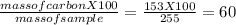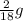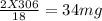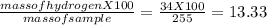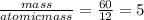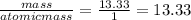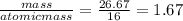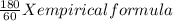Chemistry, 26.01.2020 16:31 jaymee2904p88tgh
You perform a combustion analysis on a 255 mg sample of a substance that contains only c, h, and o, and you find that 561 mg of co2 is produced, along with 306 mm of h2o.
if the substance contains only c, h, and o, what is the empirical formula
if the molar mass of the compound is 180 g/mol what is the molecular formula of the compound

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1


Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
You perform a combustion analysis on a 255 mg sample of a substance that contains only c, h, and o,...
Questions

Computers and Technology, 24.10.2020 21:00

Mathematics, 24.10.2020 21:00

Engineering, 24.10.2020 21:00

Mathematics, 24.10.2020 21:00



Mathematics, 24.10.2020 21:00


Mathematics, 24.10.2020 21:00


Mathematics, 24.10.2020 21:00

Mathematics, 24.10.2020 21:00


Computers and Technology, 24.10.2020 21:00

English, 24.10.2020 21:00





English, 24.10.2020 21:00