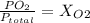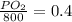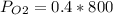Chemistry, 13.11.2019 09:31 blueflu5120
Amixture of gases with a pressure of 800.0 mm hg contains 60% nitrogen and 40% oxygen by volume. what is the partial pressure of oxygen in this mixture?
a. 140.0 mm hg
b. 320.0 mm hg
c. 373.0 mm hg
d. 480.0 mm hg

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2
You know the right answer?
Amixture of gases with a pressure of 800.0 mm hg contains 60% nitrogen and 40% oxygen by volume. wha...
Questions





Mathematics, 18.03.2021 03:00

Computers and Technology, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00


Chemistry, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

History, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00

Biology, 18.03.2021 03:00

English, 18.03.2021 03:00

English, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

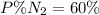
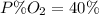
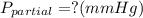
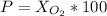
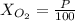
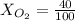
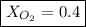
 :
: