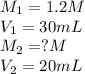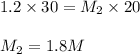Chemistry, 23.11.2019 12:31 Mariela2699
If it requires 30.0 milliliters of 1.2 molar hcl to neutralize 20.0 milliliters of naoh, what is the concentration of the naoh solution? balanced equation: naoh hcl nacl h2o 0.60 m naoh 0.80 m naoh 1.3 m naoh 1.8 m naoh

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
If it requires 30.0 milliliters of 1.2 molar hcl to neutralize 20.0 milliliters of naoh, what is the...
Questions



Geography, 01.09.2019 08:50


Mathematics, 01.09.2019 08:50

Computers and Technology, 01.09.2019 09:00

Mathematics, 01.09.2019 09:00


Mathematics, 01.09.2019 09:00

Chemistry, 01.09.2019 09:00



Mathematics, 01.09.2019 09:00

History, 01.09.2019 09:00



Mathematics, 01.09.2019 09:00



Mathematics, 01.09.2019 09:00


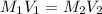
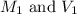 are the molarity and volume of HCl.
are the molarity and volume of HCl.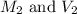 are the molarity and volume of NaOH.
are the molarity and volume of NaOH.