

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1


Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Suppose 13.00 ml of 0.100 m barium hydroxide is required to neutralize 17.00 ml of nitric acid with...
Questions


Computers and Technology, 08.04.2020 02:36

Mathematics, 08.04.2020 02:45

Chemistry, 08.04.2020 02:46

Mathematics, 08.04.2020 02:46



English, 08.04.2020 02:46

Mathematics, 08.04.2020 02:46

History, 08.04.2020 02:46

History, 08.04.2020 02:46





Mathematics, 08.04.2020 02:46

Mathematics, 08.04.2020 02:46

History, 08.04.2020 02:46


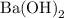 solution is
solution is 
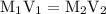
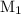 is the molarity of the first solution.
is the molarity of the first solution.
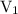 is the volume of the first solution.
is the volume of the first solution.
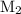 is the molarity of the second solution.
is the molarity of the second solution.
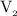 is the volume of the second solution.
is the volume of the second solution.
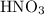 . So molarity equation becomes,
. So molarity equation becomes,
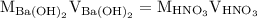 …… (1)
…… (1)
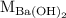 is the molarity of
is the molarity of 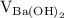 is the volume of
is the volume of 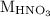 is the molarity of
is the molarity of 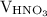 is the volume of
is the volume of 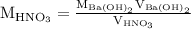 …… (2)
…… (2)




