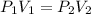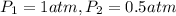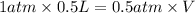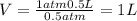Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

You know the right answer?
Aballoon filled with helium gas has a volume of 500 ml at a pressure of 1 atm. the balloon is releas...
Questions




Mathematics, 25.11.2021 06:20




Mathematics, 25.11.2021 06:20


Computers and Technology, 25.11.2021 06:20

Mathematics, 25.11.2021 06:20









Chemistry, 25.11.2021 06:20

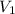 (1000 mL= 1 L)
(1000 mL= 1 L)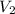
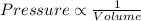 (At constant temperature)
(At constant temperature)