c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1

Chemistry, 02.09.2019 16:00 emily200705
Use the information below to answer this question
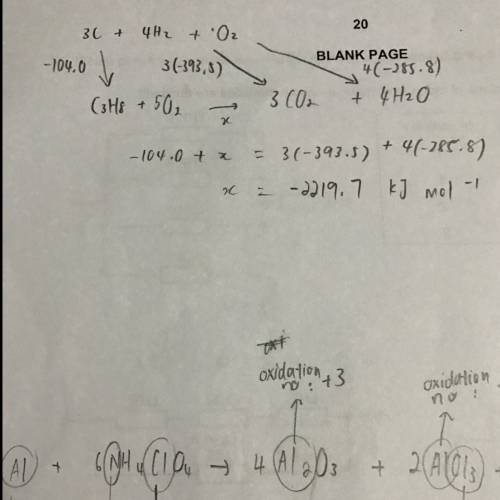
c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1
h2(g) + o2(g) → h2o(l) ∆h = −285.8 kj mol−1
3c(s) + 4h2(g) → c3h8(g) ∆h = −104.0 kj mol−1
4c(s) + 5h2(g) → c4h10(g) ∆h = −125.2 kj mol−1
the value in kj mol−1
for the enthalpy of combustion of propane is

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Dwayne filled a small balloon with air at 298.5 k. he put the balloon into a bucket of water, and the water level in the bucket increased by 0.54 liter. if dwayne puts the balloon into a bucket of ice water at 273.15 k and waits for the air inside the balloon come to the same temperature, what will the volume of the balloon be? assume the pressure inside the balloon doesn’t change. type the correct answer in the box. express your answer to the correct number of significant figures. the volume of the balloon at 273.15 k is liters.
Answers: 2

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2
You know the right answer?
Use the information below to answer this question
c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1
c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1
Questions


Mathematics, 06.05.2020 21:14


Mathematics, 06.05.2020 21:14

Mathematics, 06.05.2020 21:14


Biology, 06.05.2020 21:14


Mathematics, 06.05.2020 21:14


Mathematics, 06.05.2020 21:14

Mathematics, 06.05.2020 21:14




Mathematics, 06.05.2020 21:14