
Chemistry, 01.09.2019 23:20 kenishajohnson116
Which solute, an electrolyte or a nonelectrolyte, has the greater effect on the boiling point when a given amount of each solute is dissolved in the same mass of water?
a)the nonelectrolyte does because it disperses into molecules.
b)the electrolyte does because it disperses into molecules.
c)the nonelectrolyte does because it dissociates into ions.
d)the electrolyte does because it dissociates into ions.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1


Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2
You know the right answer?
Which solute, an electrolyte or a nonelectrolyte, has the greater effect on the boiling point when a...
Questions


Mathematics, 22.08.2019 02:00

Mathematics, 22.08.2019 02:00

Physics, 22.08.2019 02:00

Mathematics, 22.08.2019 02:00


Business, 22.08.2019 02:00



Social Studies, 22.08.2019 02:00

History, 22.08.2019 02:00


Mathematics, 22.08.2019 02:00


History, 22.08.2019 02:00

Biology, 22.08.2019 02:00


Social Studies, 22.08.2019 02:00

Mathematics, 22.08.2019 02:00

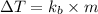
 =Elevation in boiling point
=Elevation in boiling point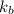 =molal boiling point constant
=molal boiling point constant

