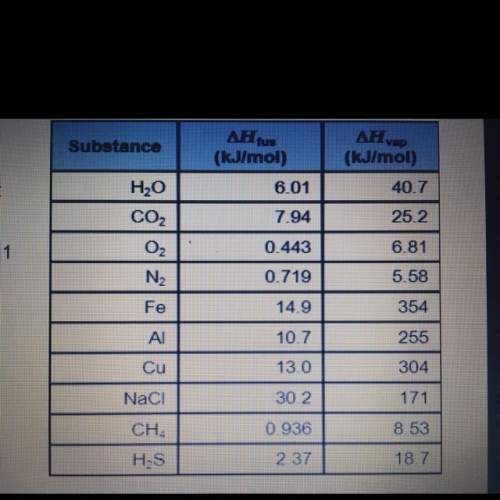
Use the table on the right to calculate each required quantity.
the quantity of heat req...

Use the table on the right to calculate each required quantity.
the quantity of heat required to melt 175.0 g cu: kj
the mass of a sample of nacl that requires 450.1 kj of heat to melt fully: g
the identity of a substance that releases 21.2 kj of energy when 1.42 mol freezes:

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
Questions


Mathematics, 22.03.2021 23:00



Mathematics, 22.03.2021 23:00


Mathematics, 22.03.2021 23:00

Mathematics, 22.03.2021 23:00

Spanish, 22.03.2021 23:00




Mathematics, 22.03.2021 23:00

Mathematics, 22.03.2021 23:00





Mathematics, 22.03.2021 23:00

Mathematics, 22.03.2021 23:00



