
The decomposition of calcium carbonate, caco3(s) --> cao(s) + co2(g), has the following values for free energy and enthalpy at 25.0°c.
g = 130.5 kj/mol
h = 178.3 kj/mol
what is the entropy of the reaction? use g = h – ts.
a. -160.3 j/(mol. k)
b. -47.8 j/(mol. k)
c. 160.3 j/(mol. k)
d. 1,912 j/(mol. k)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 08:50
Why are enzymes important to cells? they bring about chemical reactions. they provide structural support. they form the two layers of membranes. they store large quantities of energy.
Answers: 2
You know the right answer?
The decomposition of calcium carbonate, caco3(s) --> cao(s) + co2(g), has the following values f...
Questions



History, 19.07.2019 21:30




Advanced Placement (AP), 19.07.2019 21:30

Computers and Technology, 19.07.2019 21:30




Physics, 19.07.2019 21:30


Social Studies, 19.07.2019 21:30


History, 19.07.2019 21:30




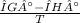
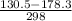 = 0.1603kJ/(mol.K) = 160.3kJ/(mol.K)
= 0.1603kJ/(mol.K) = 160.3kJ/(mol.K) 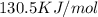
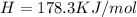
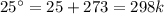
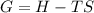
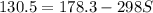

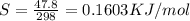
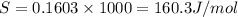 (1KJ=1000J)
(1KJ=1000J)

