
Chemistry, 27.09.2019 04:00 jorfos7683
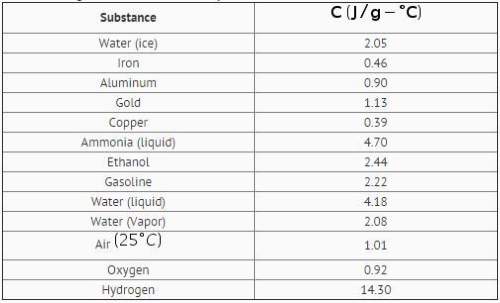
Two hundred grams of a substance requires 9.76kj of heat to raise its temperature from 25 c to 45 c. it has a specific heat of 2.44j/g. use the table to identify the substance.
a ethanol
b gasoline
c ice
d air
i think it's gasoline!

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3
You know the right answer?
Two hundred grams of a substance requires 9.76kj of heat to raise its temperature from 25 c to 45 c....
Questions




Social Studies, 15.12.2020 03:00

Mathematics, 15.12.2020 03:00

Social Studies, 15.12.2020 03:00

History, 15.12.2020 03:00

History, 15.12.2020 03:00

English, 15.12.2020 03:00



Mathematics, 15.12.2020 03:00

Mathematics, 15.12.2020 03:00

World Languages, 15.12.2020 03:00


Health, 15.12.2020 03:00


Mathematics, 15.12.2020 03:00


English, 15.12.2020 03:00



