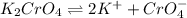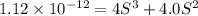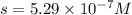Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
Silver chromate is sparingly soluble in aqueous solutions. the ksp of ag2cro4 is 1.12× 10–12. what i...
Questions


Mathematics, 16.10.2020 06:01





Mathematics, 16.10.2020 06:01

Mathematics, 16.10.2020 06:01

Chemistry, 16.10.2020 06:01

History, 16.10.2020 06:01


Biology, 16.10.2020 06:01

Geography, 16.10.2020 06:01

Social Studies, 16.10.2020 06:01

Mathematics, 16.10.2020 06:01

English, 16.10.2020 06:01

Social Studies, 16.10.2020 06:01



English, 16.10.2020 06:01

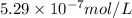
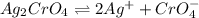
![K_{sp}=[Ag^{+}]^2[CrO_4^{-}]](/tpl/images/0248/2494/607c1.png)

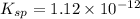
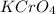 is written as:
is written as: