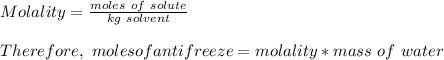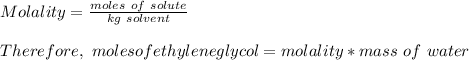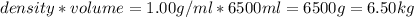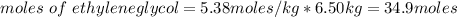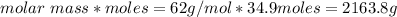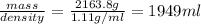Chemistry, 26.09.2019 06:00 inglehailey
How many liters of the antifreeze ethylene glycol [ch2(oh)ch2(oh)] would you add to a car radiator containing 6.50 l of water if the coldest winter temperature in your area is -10.°c? (the density of ethylene glycol is 1.11 g/ml. assume the density of water at -10.°c is 1.00 g/ml.)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
How many liters of the antifreeze ethylene glycol [ch2(oh)ch2(oh)] would you add to a car radiator c...
Questions

Chemistry, 12.05.2021 05:20

Mathematics, 12.05.2021 05:20






History, 12.05.2021 05:20

Mathematics, 12.05.2021 05:20


Mathematics, 12.05.2021 05:20

Spanish, 12.05.2021 05:20


Chemistry, 12.05.2021 05:30

Mathematics, 12.05.2021 05:30


History, 12.05.2021 05:30


Mathematics, 12.05.2021 05:30

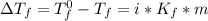
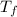 = freezing pt of solution = -10.0 C
= freezing pt of solution = -10.0 C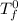 = freezing pt of pure solvent = 0 C
= freezing pt of pure solvent = 0 C![[0-(-10.0)] C= 1*(1.86 C/m) *( m)\\\\m = 5.38](/tpl/images/0263/9231/f27db.png)