Chemistry, 17.12.2019 09:31 dextor1606
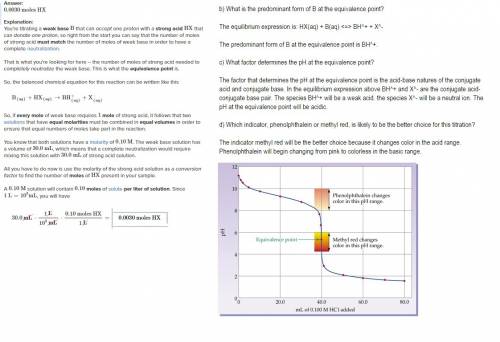
Assume that 30.0 ml of a 0.10 m solution of a weak base b that accepts one proton is titrated with a 0.10 m solution of a monoprotic strong acid hx. (a) how many moles of hx have been added at the equivalence point? (b) what is the predominant form of b at the equivalence point? (c) what factor determines the ph at the equivalence point? (d) which indicator, phenolphthalein or methyl red, is likely to be the better choice for this titration?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
Assume that 30.0 ml of a 0.10 m solution of a weak base b that accepts one proton is titrated with a...
Questions


Health, 02.04.2021 22:10

Physics, 02.04.2021 22:10





Health, 02.04.2021 22:10


Health, 02.04.2021 22:10



Mathematics, 02.04.2021 22:10




Mathematics, 02.04.2021 22:10