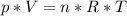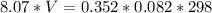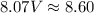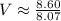Chemistry, 12.10.2019 05:00 sallylove03
Determine the volume occupied by 0.352 mole of a gas at 25⁰c if the pressure is 81.8 kpa.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
You know the right answer?
Determine the volume occupied by 0.352 mole of a gas at 25⁰c if the pressure is 81.8 kpa....
Questions



Mathematics, 11.02.2021 02:50



Mathematics, 11.02.2021 02:50

Mathematics, 11.02.2021 02:50

Mathematics, 11.02.2021 02:50


Social Studies, 11.02.2021 02:50


Health, 11.02.2021 02:50


Mathematics, 11.02.2021 02:50




Mathematics, 11.02.2021 02:50

History, 11.02.2021 02:50

Chemistry, 11.02.2021 02:50