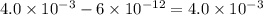The equation for the ph of a substance is ph = –log[h+], where h+ is the concentration of hydrogen ions. a basic solution has a ph of 11.2. an acidic solution has a ph of 2.4. what is the approximate difference in the concentration of hydrogen ions between the two solutions? a) 1.6*10^-9 b) 4.0*10^-3 c)6.7*10^-1 d)1.6*10^-11

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
The equation for the ph of a substance is ph = –log[h+], where h+ is the concentration of hydrogen i...
Questions

Chemistry, 17.11.2020 19:20

Biology, 17.11.2020 19:20

Mathematics, 17.11.2020 19:20




Biology, 17.11.2020 19:20

Social Studies, 17.11.2020 19:20


English, 17.11.2020 19:20



Physics, 17.11.2020 19:20

History, 17.11.2020 19:20

Physics, 17.11.2020 19:20


Mathematics, 17.11.2020 19:20


Mathematics, 17.11.2020 19:20

Engineering, 17.11.2020 19:20

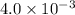
![pH=-\log [H^+]](/tpl/images/0451/5539/37e81.png)
![pH=-\log[H^+]](/tpl/images/0451/5539/cf945.png)
![11.2=-\log[H^+]](/tpl/images/0451/5539/fa6ad.png)
![[H^+]=6\times 10^{-12}M](/tpl/images/0451/5539/5538b.png)
![2.4=-\log[H^+]](/tpl/images/0451/5539/156c9.png)
![[H^+]=4\times 10^{-3}M](/tpl/images/0451/5539/9bba1.png)
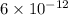 is very less as comapred to
is very less as comapred to