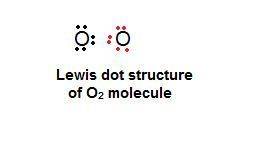
Chemistry, 13.12.2019 17:31 jladinosolarsee
What best explains how two oxygen atoms, each with six valence electrons, can bond with each other?
one atom can lose two electrons so that the other atom can gain them and have eight valence electrons.
one atom can lose four electrons to the environment so that a total of eight valence electrons remains.
each atom can share two electrons with the other so that each atom has eight valence electrons.
each atom can lose two electrons so that there is a total of eight valence electrons between the atoms.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
What best explains how two oxygen atoms, each with six valence electrons, can bond with each other?...
Questions

History, 16.02.2021 23:30

Mathematics, 16.02.2021 23:30


Mathematics, 16.02.2021 23:30

Law, 16.02.2021 23:30

Business, 16.02.2021 23:30

Social Studies, 16.02.2021 23:30


Chemistry, 16.02.2021 23:30

Mathematics, 16.02.2021 23:30




Mathematics, 16.02.2021 23:30


Mathematics, 16.02.2021 23:30


Computers and Technology, 16.02.2021 23:30

Mathematics, 16.02.2021 23:30

Biology, 16.02.2021 23:30

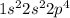
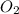 molecule is given in the image attached.
molecule is given in the image attached.