
Chemistry, 28.01.2020 21:58 alaina3792
When a person has excess stomach acid, he or she may ingest some sodium bicarbonate. the rationale for doing this is that the sodium bicarbonate is:
a base and will neutralize the excess acid
an acid and will weaken the excess acid
neutral and will dilute the excess acid
it will make the person thirsty; they will drink water and dilute the excess acid.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 05:30
Astudent made the lewis dot diagram of a compound as shown. mg is written with two dots shown on its top. an o is written on each side of mg. each o has six dots around it. an arrow is shown from one dot on mg toward the vacant space around the o on the right. another arrow is shown from the other dot on mg toward the vacant space around the o on the left. the title of the art is students lewis dot model. what is the error in the lewis dot diagram? an o atom should transfer all its six electrons to mg because the formula is mgo. both electrons of mg should be transferred to one o atom because the formula is mgo. the electrons should be transferred from each o atom to mg because mg has fewer electrons. the number of dots around mg should be four because it has to transfer two electrons to each o.
Answers: 2

Chemistry, 23.06.2019 08:30
Sand is more likely than shale to preserve fossils. true false
Answers: 2
You know the right answer?
When a person has excess stomach acid, he or she may ingest some sodium bicarbonate. the rationale f...
Questions

Mathematics, 16.11.2020 03:30

Biology, 16.11.2020 03:30


Mathematics, 16.11.2020 03:40

History, 16.11.2020 03:40



Biology, 16.11.2020 03:40

Mathematics, 16.11.2020 03:40


Mathematics, 16.11.2020 03:40


Mathematics, 16.11.2020 03:40


Biology, 16.11.2020 03:40

Mathematics, 16.11.2020 03:40

Mathematics, 16.11.2020 03:40


English, 16.11.2020 03:40

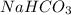 . It is basic in nature as it dissociates to give sodium and bicarbonate ions.
. It is basic in nature as it dissociates to give sodium and bicarbonate ions. 

