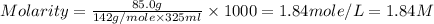Chemistry, 07.01.2020 11:31 lovelyheart5337
What is the molarity of a solution that contains 85.0 grams of na2so4 in 325 milliliters of solution? (the mass of one mole of na2so4 is 142 grams.)
0.195 m
0.599 m
1.84 m
6.22 m

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2


Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
What is the molarity of a solution that contains 85.0 grams of na2so4 in 325 milliliters of solution...
Questions

Business, 09.12.2020 02:10




Mathematics, 09.12.2020 02:10

English, 09.12.2020 02:10



English, 09.12.2020 02:10


Mathematics, 09.12.2020 02:10




Mathematics, 09.12.2020 02:10




Mathematics, 09.12.2020 02:10

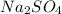 = 85.0 g
= 85.0 g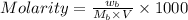
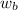 = mass of solute
= mass of solute 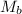 = molar mass of solute
= molar mass of solute 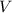 = volume of solution in ml
= volume of solution in ml