

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

You know the right answer?
Given the unbalanced equation: fe2o3 + co --> fe + co2, when the equation is correctly balanced...
Questions



Mathematics, 14.10.2020 01:01



Computers and Technology, 14.10.2020 01:01

Health, 14.10.2020 01:01

Mathematics, 14.10.2020 01:01

Computers and Technology, 14.10.2020 01:01

Mathematics, 14.10.2020 01:01



Mathematics, 14.10.2020 01:01

Mathematics, 14.10.2020 01:01

Mathematics, 14.10.2020 01:01




Business, 14.10.2020 01:01

Physics, 14.10.2020 01:01

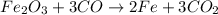
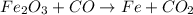
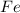 and the coefficient '3' put before the
and the coefficient '3' put before the 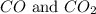 we get the balanced chemical equation.
we get the balanced chemical equation.

