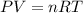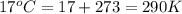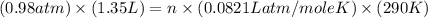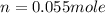Chemistry, 30.09.2019 02:30 19thomasar
Asapp
determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. what is the number of moles present?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:20
Amixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 k where they react to form so3(g). if the vessel contains 0.669 atm so2(g), 0.395 atm o2(g), and 0.0851 atm so3(g) after the system has reached equilibrium, what is the equilibrium constant kp for the reaction: 2 so2(g) o2(g) ⇌ 2 so3(g)
Answers: 3


Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
Asapp
determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. what...
determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. what...
Questions

Mathematics, 27.07.2021 14:00

English, 27.07.2021 14:00



Mathematics, 27.07.2021 14:00

Mathematics, 27.07.2021 14:00

Mathematics, 27.07.2021 14:00

Mathematics, 27.07.2021 14:00


Mathematics, 27.07.2021 14:00





Biology, 27.07.2021 14:00

Mathematics, 27.07.2021 14:00

English, 27.07.2021 14:00



Computers and Technology, 27.07.2021 14:00