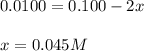10.0 ml of a 0.100 mol l–1 solution of a metal ion m2+ is mixed with 10.0 ml of a 0.100 mol l–1 solution of a substance l. the following equilibrium is established:
m2+(aq) + 2l(aq) picture ml22+(aq)
at equilibrium the concentration of l is found to be 0.0100 mol l–1. what is the equilibrium concentration of ml22+, in mol l–1?
someone me

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
A32 year old immigrant from a patriarchal country is giving birth. as she is delivering the baby, she tearfully confesses to her doctor that this is her 4th child and she simply cannot handle any more children. she tells the doctor that her husband refuses to use contraception or allow her to, and she begs her doctor to tie her tubes and not tell her husband. the doctor complies. was hipaa violated? why or why not?
Answers: 3

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1

You know the right answer?
10.0 ml of a 0.100 mol l–1 solution of a metal ion m2+ is mixed with 10.0 ml of a 0.100 mol l–1 solu...
Questions


Mathematics, 06.05.2020 01:13


History, 06.05.2020 01:13

History, 06.05.2020 01:13



Mathematics, 06.05.2020 01:13

Mathematics, 06.05.2020 01:13

Mathematics, 06.05.2020 01:13


Mathematics, 06.05.2020 01:13

Chemistry, 06.05.2020 01:13

English, 06.05.2020 01:13



History, 06.05.2020 01:13

Mathematics, 06.05.2020 01:13

History, 06.05.2020 01:13

Physics, 06.05.2020 01:13

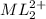 at equilibrium is 0.045 M.
at equilibrium is 0.045 M.![[M^{2+}]_{initial}=0.100M](/tpl/images/0204/7051/8debc.png)
![[L]_{initial}=0.100M](/tpl/images/0204/7051/9e7cd.png)
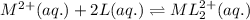
![[L]_{eqllm}=0.0100M](/tpl/images/0204/7051/18682.png)