In the first law of thermodynamics, △e = q-w, what does △e stand for?
a. the work done...

Chemistry, 09.01.2020 08:31 keigleyhannah30
In the first law of thermodynamics, △e = q-w, what does △e stand for?
a. the work done by the piston
b. the internal energy of the system
c. the heat added to the system
d. the change in the internal energy of the system

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
Questions

English, 04.11.2019 10:31

Mathematics, 04.11.2019 10:31

Biology, 04.11.2019 10:31

Mathematics, 04.11.2019 10:31

Mathematics, 04.11.2019 10:31


History, 04.11.2019 10:31

History, 04.11.2019 10:31





History, 04.11.2019 10:31

Biology, 04.11.2019 10:31

French, 04.11.2019 10:31

History, 04.11.2019 10:31


Mathematics, 04.11.2019 10:31


Chemistry, 04.11.2019 10:31

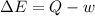
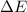 represents the internal energy of the system.
represents the internal energy of the system.

