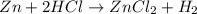When zinc is placed in hydrochloric acid, a gas is produced. when the gas is collected in a tube and a lit match is placed in the tube, a pop is heard. this is a test for hydrogen gas. what can be concluded from this observations. a) a change in state has occurred. b) the zinc metal was turned into hydrogen gas. c) a chemical change has occurred producing the hydrogen gas. d) a physical change has occurred producing the hydrogen gas.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
When zinc is placed in hydrochloric acid, a gas is produced. when the gas is collected in a tube and...
Questions

English, 07.10.2020 08:01

History, 07.10.2020 08:01

Biology, 07.10.2020 08:01

Mathematics, 07.10.2020 08:01

Advanced Placement (AP), 07.10.2020 08:01

English, 07.10.2020 08:01

Biology, 07.10.2020 08:01



Biology, 07.10.2020 08:01

Biology, 07.10.2020 08:01


Mathematics, 07.10.2020 08:01

Health, 07.10.2020 08:01

Biology, 07.10.2020 08:01

History, 07.10.2020 08:01




History, 07.10.2020 08:01