
Chemistry, 17.11.2019 02:31 aaronw3743
Using the equation, 4fe + 3o2 imported asset 2fe2o3, if 8 moles of iron and oxygen from the air were available, how many moles of iron (iii) oxide would be produced?
4 moles
5 moles
6 moles
8 moles

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
Using the equation, 4fe + 3o2 imported asset 2fe2o3, if 8 moles of iron and oxygen from the air were...
Questions

Advanced Placement (AP), 06.11.2021 01:40








Mathematics, 06.11.2021 01:40

Chemistry, 06.11.2021 01:40










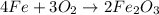
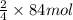 of iron oxide.
of iron oxide.

