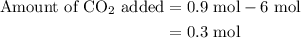Chemistry, 30.01.2020 05:57 Queiao4088
An equilibrium mixture contains 0.600 mol of each of the products (carbon dioxide and hydrogen gas) and 0.200 mol of each of the reactants (carbon monoxide and water vapor) in a 1.00-l container. how many moles of carbon dioxide would have to be added at constant temperature and volume to increase the amount of carbon monoxide to 0.300 mol once equilibrium has been reestablished?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1


Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1
You know the right answer?
An equilibrium mixture contains 0.600 mol of each of the products (carbon dioxide and hydrogen gas)...
Questions

Social Studies, 19.01.2021 16:10


Mathematics, 19.01.2021 16:10


Chemistry, 19.01.2021 16:10


Mathematics, 19.01.2021 16:10

English, 19.01.2021 16:10

Biology, 19.01.2021 16:10

Mathematics, 19.01.2021 16:10

Mathematics, 19.01.2021 16:10


Arts, 19.01.2021 16:10

Mathematics, 19.01.2021 16:10



Mathematics, 19.01.2021 16:10


Business, 19.01.2021 16:10

English, 19.01.2021 16:10

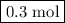 of
of 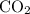 are added so as to increase the amount of carbon monoxide to 0.3 mol.
are added so as to increase the amount of carbon monoxide to 0.3 mol.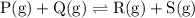
![{\text{K}}=\dfrac{{\left[ {\text{R}} \right]\left[ {\text{S}}\right]}}{{\left[{\text{P}}\right]\left[ {\text{Q}} \right]}}](/tpl/images/0484/7673/9b899.png)
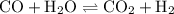
![{\text{K = }}\dfrac{{\left[ {{\text{C}}{{\text{O}}_{\text{2}}}} \right]\left[{{{\text{H}}_{\text{2}}}} \right]}}{{\left[ {{\text{CO}}}\right]\left[{{{\text{H}}_2}{\text{O}}} \right]}}](/tpl/images/0484/7673/6dcad.png) .......(1)
.......(1)![\left[{{\text{C}}{{\text{O}}_{\text{2}}}}\right]](/tpl/images/0484/7673/9014c.png) is the concentration of carbon dioxide.
is the concentration of carbon dioxide.
![\left[{{{\text{H}}_{\text{2}}}}\right]](/tpl/images/0484/7673/340fe.png) is the concentration of hydrogen.
is the concentration of hydrogen.
![\left[{{\text{CO}}}\right]](/tpl/images/0484/7673/d6da7.png) is the concentration of carbon monoxide.
is the concentration of carbon monoxide.
![\left[{{{\text{H}}_2}{\text{O}}}\right]](/tpl/images/0484/7673/62a9e.png) is the concentration of water.
is the concentration of water.
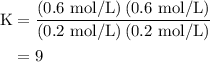
![\left[{{\text{C}}{{\text{O}}_{\text{2}}}}\right]=\dfrac{{{\text{K}}\left( {\left[{{\text{CO}}} \right]\left[{{{\text{H}}_2}{\text{O}}}\right]}\right)}}{{\left[{{{\text{H}}_{\text{2}}}} \right]}}](/tpl/images/0484/7673/f6940.png) ......(2)
......(2)![\begin{aligned}\left[ {{\text{C}}{{\text{O}}_{\text{2}}}}\right]&= \frac{{{\text{9}}\left( {{\text{0}}{\text{.3 mol/L}}}\right)\left({{\text{0}}{\text{.2 mol/L}}}\right)}}{{{\text{0}}{\text{.6 mol/L}}}}\\&= 0.{\text{9 mol/L}}\\\end{aligned}](/tpl/images/0484/7673/95ec6.png)